BioNTech-Pfizer COVID-19 Vaccine Candidate Efficacy - Part 3
Nov 13, 2020 · 239 words · 2 minutes read
My last two posts (here and here) looked at the BioNTech-Pfizer vaccine candidate efficacy. This post continues exploring what we know about the BioNTech-Pfizer vaccine candidate.
In the previous posts I looked at posterior distributions of the vaccine candidate’s efficacy. Here I look at the posterior distributions of the rate parameter of the binomial distributions in the model. The model fits a binomial for each group, the treatment and control, in which the rate, theta, is drawn from a beta distribution. The rate parameter, theta, represents the probability of a confirmed COVID-19 case for that group. For the beta distributions, I use the shape parameters specified in the study protocol (0.700102, 1).
The plot below shows the theta posterior distributions for the treatment and control groups.
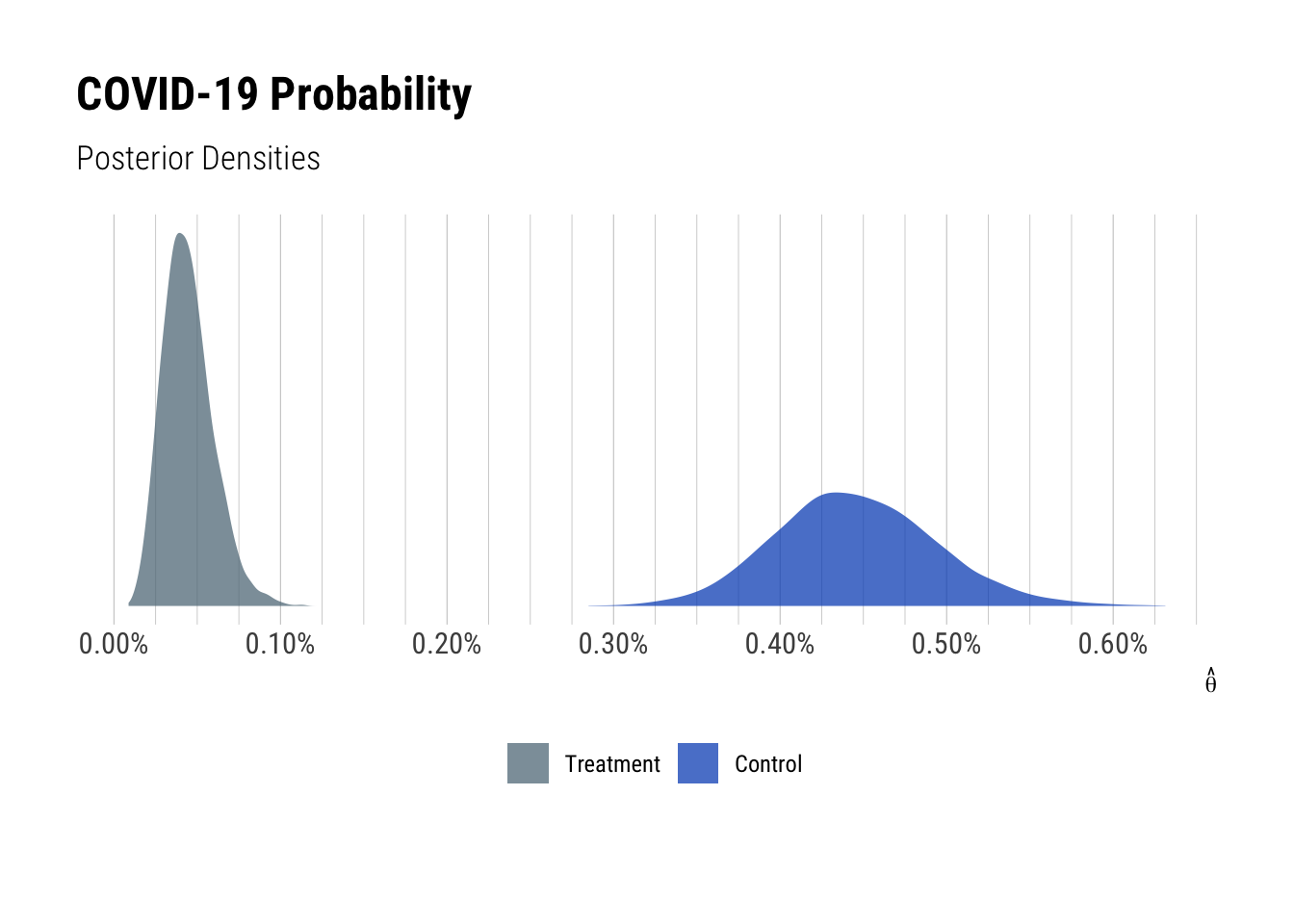
The separation of the posterior distributions demonstrates the probability of contracting COVID-19 is differs between the treatment and control groups. As the COVID-19 probabilities are lower within the treatment distribution, there is clear evidence of the vaccine candidate’s efficacy. This is consistent with generated values of the efficacy but viewing the posterior distributions for theta shows the disaggregated uncertainty. In this case, the posterior interval spread for the control group is larger than the treatment group which indicates a greater range of theta values could have produced the number of confirmed cases in that group. Comparatively, the posterior interval for the treatment group could only be produced by a narrower range of probabilities.