BioNTech-Pfizer COVID-19 Vaccine Candidate Efficacy - Part 4
Nov 18, 2020 · 271 words · 2 minutes read
Today Pfizer and BioNTech released findings from their latest analysis of their COVID-19 vaccine candidate. The press release is based on more recently available data. There have been 41,135 participants who have received both doses and 170 confirmed cases of COVID-19; 162 cases occurred in the placebo group while 8 cases occurred in the treatment group.
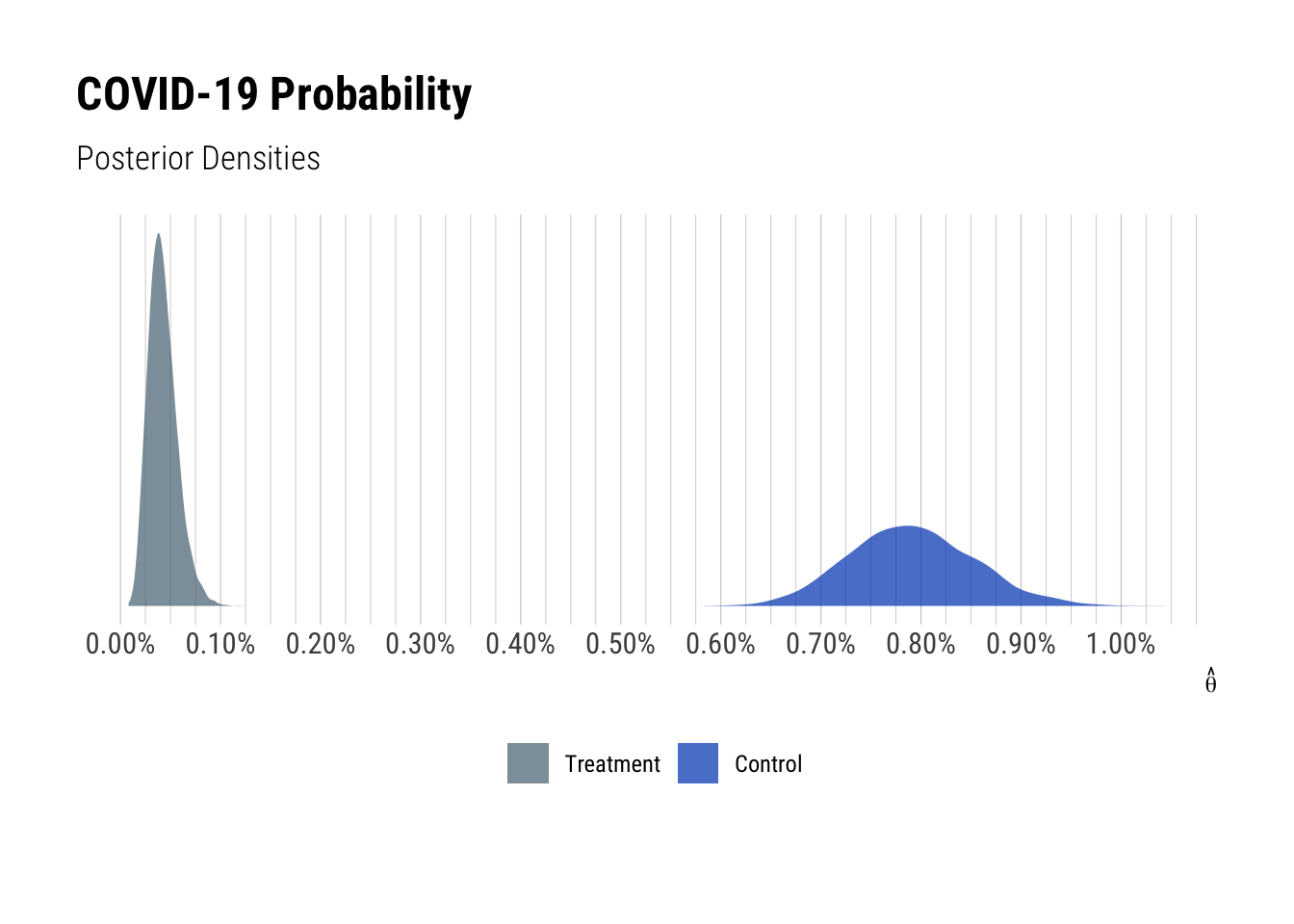
Based on this information, I re-ran the model from my earlier post. The plots below show the posterior distribution for vaccine candidate efficacy and the group probabilities of COVID-19.
## Warning: It is deprecated to specify `guide = FALSE` to remove a guide. Please
## use `guide = "none"` instead.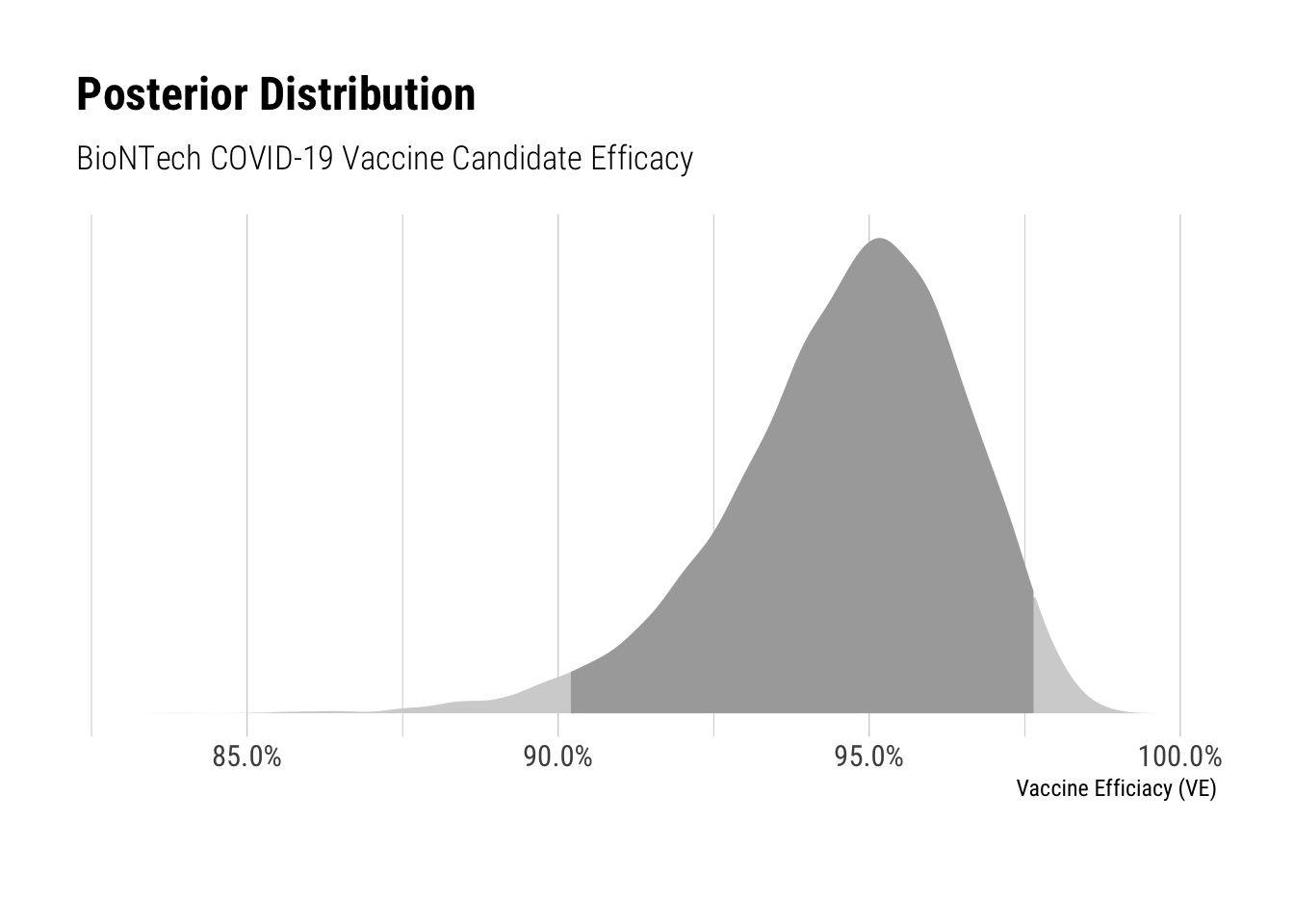
## Inference for Stan model: 88c4792d2ae31f0d91937cb65a7a3c38.
## 4 chains, each with iter=6000; warmup=3000; thin=1;
## post-warmup draws per chain=3000, total post-warmup draws=12000.
##
## mean se_mean sd 2.5% 50% 97.5% n_eff Rhat
## theta1 0.000 0.000 0.000 0.000 0.000 0.001 9016 1
## theta2 0.008 0.000 0.001 0.007 0.008 0.009 9589 1
## efficacy 0.946 0.000 0.019 0.902 0.948 0.976 9061 1
## lp__ -1026.764 0.014 1.028 -1029.472 -1026.443 -1025.772 5087 1
##
## Samples were drawn using NUTS(diag_e) at Thu Jan 6 18:49:48 2022.
## For each parameter, n_eff is a crude measure of effective sample size,
## and Rhat is the potential scale reduction factor on split chains (at
## convergence, Rhat=1).The vaccine candidate efficacy has a 95% credible interval from 90.2% to 97.6%, which looks quite good. The chance of a confirmed COVID-19 case within the treatment group has 95% credible interval from 0.02% to 0.07%, compared to 0.67% to 0.92% for the control group. Thus the results are looking excellent.